TL;DR
- Nearly 60% of pharmaceutical drug launches underperform in their first year, even with strong clinical data.
- These failures follow repeating patterns, not isolated execution mistakes.
- The root issue is an early gap in real patient journey understanding, not a lack of effort or data.
- Marketing teams often inherit this gap late, limiting their ability to course-correct.
- Continuous patient insight helps teams align earlier, differentiate more clearly, and reduce launch risk.
This article summarizes key findings from mama health’s 2026 Commercial Drug Launch Analysis.
Download the full report: Commercial Drug Launches: Why 60% Fail
Why do so many pharmaceutical drug launches underperform?
Because commercial strategies are finalized before teams fully understand how patients actually experience the journey to treatment.
Across the pharmaceutical industry, roughly six out of ten new drug launches fail to meet first-year expectations. This pattern appears consistently across therapeutic areas, company sizes, and regions.
What makes this underperformance persistent is not a lack of preparation. Most launches are supported by:
- years of clinical development
- extensive market research
- significant commercial investment
Yet soon after approval, momentum slows. Awareness does not translate into sustained uptake, and early gaps prove difficult to recover from.
The issue is not that launches fail in many different ways.
They fail in remarkably similar ways.
What actually drives these repeated launch failures?
Restricted access, weak differentiation, and slow uptake are symptoms of the same upstream problem.
Post-launch reviews typically cite familiar causes: payer resistance, cautious physicians, or unclear positioning. The 2026 mama health analysis confirms these factors matter, but it also shows they rarely originate where teams think they do.
In practice, these challenges emerge when launch decisions are made without a shared, grounded understanding of:
- how patients reach diagnosis
- where they encounter friction in access
- what trade-offs matter in daily life
- why they hesitate, delay, or discontinue
When these realities are only discovered after launch, teams are forced into reactive mode. By then, positioning, access strategy, and messaging are already difficult to change.
Why does traditional pharma research miss this early?
Because most patient insight arrives as static snapshots, not continuous signals.
Pre-launch research remains essential, but it is often:
- episodic rather than ongoing
- slow to reflect changing behavior
- detached from real-life context
As a result, insights tend to validate assumptions instead of challenging them. Teams frequently identify issues such as treatment hesitation or access friction only once they appear in lagging indicators, like claims or sales data .
At that stage, the cost of adjustment is high and the opportunity to shape early momentum has passed.
What does this mean for pharma marketing teams?
Marketing teams are rarely failing at execution; they are constrained by the inputs they receive.
Marketing is expected to:
- translate evidence into compelling value stories
- align messaging across stakeholders
- drive early adoption
But when patient understanding is incomplete or arrives late, marketing teams are left refining messages around assumptions rather than reality.
This explains why:
- positioning feels technically accurate but emotionally flat
- differentiation resonates in decks but not in practice
- teams struggle to align internally around one narrative
When marketing teams have access to shared, real-time patient insights early, they shift from reacting to problems to preventing them.

How does continuous patient insight change launch decision-making?
It turns patient experience into a living strategic input instead of a one-time deliverable.
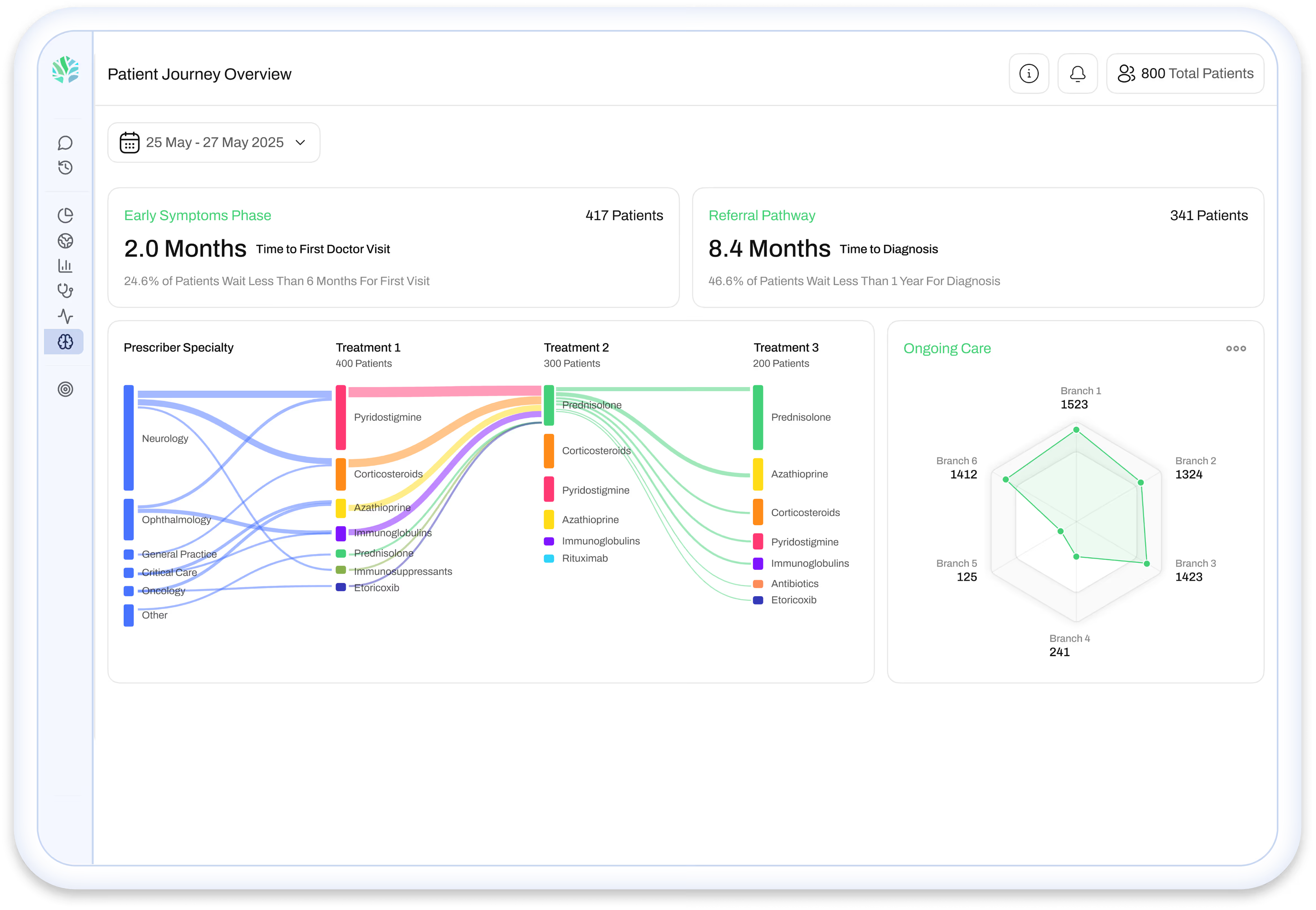
The 2026 mama health analysis highlights a clear pattern: launches perform more consistently when teams maintain ongoing visibility into how patients experience their condition and treatment pathway.
This allows teams to:
- validate positioning before scale
- surface unmet needs competitors overlook
- detect early signals of hesitation or drop-off
- align commercial, access, and medical teams around the same reality
Rather than replacing existing research, continuous insight strengthens it by adding speed, context, and continuity.
The full report explores how this approach affects decisions across launch phases and therapeutic areas.
Access the full 2026 Commercial Drug Launch Analysis
Why is this especially critical in today’s launch environment?
Because launches now unfold under greater uncertainty and scrutiny than ever before.
Post-COVID dynamics have reshaped the commercial landscape:
- reduced in-person access to healthcare professionals
- increased payer sensitivity to value
- higher expectations for real patient insights
- faster shifts in stakeholder behavior
In this environment, static launch plans age quickly. Teams that lack early, continuous patient perspective struggle to adapt without fragmenting strategy.
The analysis shows that organizations able to integrate real-time patient journey mapping are better positioned to adjust while maintaining coherence .
From launch underperformance to launch readiness
Pharmaceutical launches will always involve risk. But the persistent 60% underperformance rate is not inevitable.
The launches that outperform consistently share a common approach:
- they surface patient reality early
- they align teams around shared insight
- they ground differentiation in lived experience
- they adapt before small issues become structural problems
The 2026 mama health Commercial Drug Launch Analysis documents these patterns in depth, with concrete examples across therapy areas and launch stages.



.avif)
.avif)
%20(1)%20(1).avif)