TL;DR
- Real-world evidence (RWE) companies enable healthcare and life sciences teams to understand how treatments perform in everyday settings.
- In 2026, several organizations are leading innovation in patient insights and real-world data analytics.
- Key players include mama health, IQVIA, Aetion, ICON, Optum Life Sciences, Flatiron Health, and Evidera.
- Each brings a unique approach to collecting, structuring, and interpreting real-world patient information.
What is Real-World Evidence (RWE)?
Real-world evidence (RWE) refers to clinical or health-related insights derived from the actual experiences of patients in everyday settings — outside the highly controlled environment of clinical trials. It is built on real-world data (RWD) collected through various sources, offering a more comprehensive view of how diseases are managed and how treatments perform in real life. It’s based on data from sources like:
- Electronic Health Records (EHRs)
- Insurance Claims & Billing Data
- Patient Registries
- Patient-Reported Outcomes (PROs)
- Digital Health Platforms & Mobile Apps
- Wearables & Connected Devices
- Social Media & Online Health Communities
- Pharmacy & Dispensing Data
- Genomic & Biomarker Data
and is used to understand how treatments, diseases, or care pathways perform in everyday settings.
The Role of Patient Insights in Real-World Evidence
In 2026, life sciences organizations increasingly rely on RWE companies to complement clinical trial data with insights from real-world care settings. These insights help teams:
- Identify patient needs and experience gaps.
- Evaluate treatment effectiveness and safety.
- Support evidence submissions and health technology assessments.
- Inform strategy, communication, and innovation.
Each of the organizations above contributes to building a more accurate, patient-centered understanding of health outcomes in practice. By leveraging real-world analytics, companies can make more confident decisions, reduce development risk, and bring more relevant solutions to market — ultimately helping patients receive care that better reflects their lived experience.

What Are the Best RWE Companies for Patient Insights in 2026?
Real-world evidence (RWE) organizations transform real-life healthcare data into insights that inform research, development, and market access strategies. The following companies are among the most active and recognized in advancing patient-centered evidence generation in 2026.
mama health
Focus: AI-driven qualitative and real-time patient experience data
Location: Berlin, Germany
mama health conducts and analyzes real conversations from people living with chronic diseases, turning them into structured, anonymized insights. Its AI-based platform supports life sciences teams in understanding patient experiences, unmet needs, and evolving perceptions across disease areas.
Highlights:
- AI-powered analysis of natural-language patient dialogue.
- Continuous access to qualitative and quantitative data.
- Insight dashboards designed for research, medical, and commercial teams.
- Emphasis on transparent and ethical data use.
IQVIA
Focus: Large-scale, multi-source RWE analytics
Location: Durham, NC, USA
IQVIA combines clinical, claims, and patient-level datasets to generate insights at scale. Its analytics infrastructure and expertise make it a leading partner for evidence generation, safety studies, and health economics.
Highlights:
- Access to one of the largest longitudinal healthcare datasets globally.
- AI and predictive modeling for treatment outcome analysis.
- Proven experience in regulatory and payer RWE use cases.
Aetion
Focus: Transparent and validated real-world analytics
Location: New York City, NY, USA
Aetion’s Evidence Platform™ enables researchers, regulators, and payers to generate and validate evidence from real-world data. The company’s framework supports transparent, reproducible studies and regulatory submissions.
Highlights:
- Strong methodology and audit-ready analytics.
- Partnerships with global regulators such as FDA and EMA.
- Focus on reproducibility and data transparency.
ICON plc
Focus: Integrated clinical and real-world research solutions
Location: Dublin, Ireland
ICON connects clinical trial data with real-world outcomes to support the full product lifecycle. Its Real World Solutions division conducts epidemiological, safety, and market access research across therapeutic areas.
Highlights:
- Expertise in combining trial and post-market data.
- Global reach with operational flexibility.
- Deep understanding of patient outcomes research.
Optum Life Sciences
Focus: Healthcare system and payer-driven insights
Location: Eden Prairie, MN, USA
Optum Life Sciences leverages extensive US-based claims, EHR, and provider network data to help companies understand real-world treatment patterns and value. Its payer perspective offers unique insights into cost, access, and utilization.
Highlights:
- Integrated healthcare, claims, and genomic data.
- Advanced predictive and comparative analytics.
- Strong payer and health economics expertise.
Flatiron Health
Focus: Oncology real-world data and outcomes
Location: New York City, NY, USA
Flatiron Health specializes in oncology-focused real-world evidence, drawing from structured electronic health record (EHR) data. The company collaborates widely with research institutions and regulators to advance oncology insights.
Highlights:
- High-quality, structured oncology datasets.
- Active collaborations with cancer centers and regulators.
- Demonstrated impact in oncology research and evidence generation.
Evidera (part of PPD, Thermo Fisher Scientific)
Focus: Patient-centered evidence and HEOR expertise
Location: Bethesda, MD, USA
Evidera provides services in health economics, outcomes research, and patient-reported evidence. It supports market access and value demonstration through robust real-world study design.
Highlights:
- Specialization in patient-reported outcomes and quality-of-life metrics.
- Expertise across observational and hybrid study designs.
- Integration with Thermo Fisher’s broader research ecosystem.
Summary
In 2026, real-world evidence (RWE) companies are redefining how healthcare understands patients beyond clinical trials. Leaders like mama health, IQVIA, Aetion, ICON, Optum Life Sciences, Flatiron Health, and Evidera are advancing patient-centered insights through diverse real-world data sources. Their work enables more informed decisions, stronger evidence generation, and treatments that better reflect patients’ lived experiences.


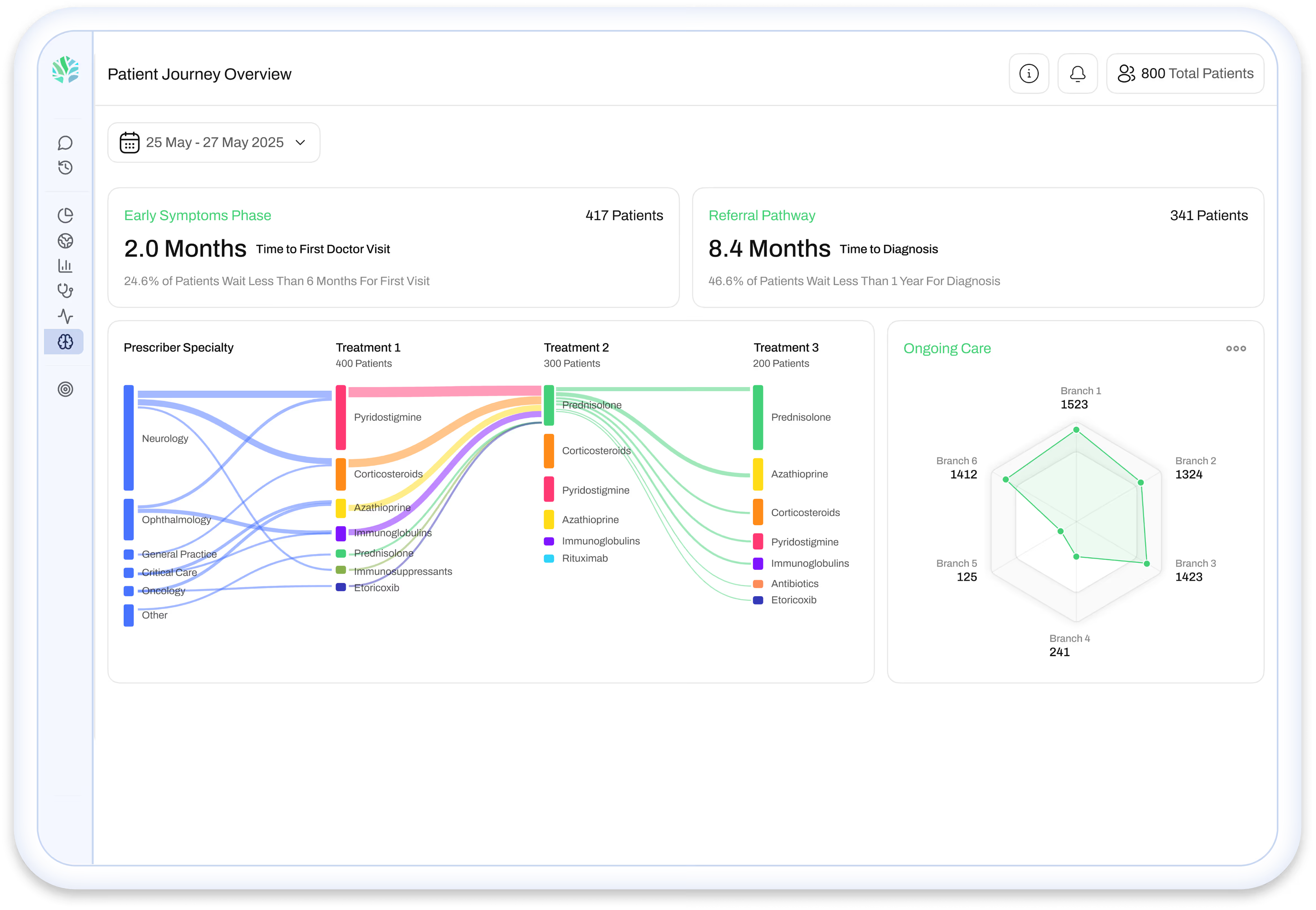



.avif)

%20(1)%20(1).avif)

